truqap moa|Capivasertib : Baguio TRUQAP in combination with fulvestrant is indicated for the treatment of adult patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2) . Welcome to the Idaho Gateway for EMS (IGEMS) license management system for. EMS Personnel and Agencies in the State of Idaho. With your help, we can streamline processes within IGEMS to make it the most user-friendly license management system. We ask that you help us identify issues by communicating those to us as you experience them.If in the future we will need some hard-to-find electronic components, we will certainly get them at Utmel electronic. Date of experience: May 15, 2023. Reply from Utmel. . Anyone can write a Trustpilot review. People who write reviews have ownership to edit or delete them at any time, and they’ll be displayed as long as an account is active.
PH0 · Truqap: Uses, Dosage, Side Effects, Warnings
PH1 · Truqap (capivasertib) dosing, indications, interactions, adverse
PH2 · TRUQAP Full Prescribing Information
PH3 · Selective AKT kinase inhibitor capivasertib in combination with
PH4 · Mechanism of Action (MOA)
PH5 · Capivasertib: Uses, Interactions, Mechanism of Action
PH6 · Capivasertib: First Approval
PH7 · Capivasertib in Hormone Receptor–Positive Advanced Breast
PH8 · Capivasertib
Powered by Forms Version: 2024-08-15T22:25:08.246+08:00
truqap moa*******TRUQAP in combination with fulvestrant is indicated for the treatment of adult patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2) .Capivasertib, sold under the brand name Truqap, is an anti-cancer medication used for the treatment of breast cancer. It is taken by mouth. The most common adverse reactions include diarrhea, cutaneous adverse reactions, increased random glucose, decreased lymphocytes, decreased hemoglobin, increased fasting glucose, nausea, fatigue, decreased leukocyte.TRUQAP, in combination with fulvestrant, is indicated for the treatment of adult patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, locally advanced or metastatic breast cancer with one or moreNob 21, 2023 — Truqap (capivasertib) is a kinase inhibitor used to treat breast cancer that is HR-positive or HER2-negative and has an abnormal PIK3CA, AKT1, or PTEN gene. It is .May 31, 2023 — Capivasertib is an orally bioavailable, small-molecule inhibitor of all three AKT isoforms. 17 Capivasertib inhibited AKT in preclinical models, resulting in .Capivasertib (Truqap™) is an orally available, small-molecule pan-AKT inhibitor being developed by AstraZeneca for the treatment of various cancers, including breast and .
Peb 23, 2024 — Capivasertib (Truqap™) is an orally available, small-molecule pan-AKT inhibitor being developed by AstraZeneca for the treatment of various cancers, including .Abr 16, 2021 — Five to ten percent of ER+ metastatic breast cancer (MBC) tumors harbor somatic PTEN mutations. Loss of function of this tumor-suppressor gene defines a .
Capivasertib, sold under the brand name Truqap, is an anti-cancer medication used for the treatment of breast cancer. [4] [7] It is taken by mouth.[4]The most common adverse reactions include diarrhea, cutaneous adverse reactions, increased random glucose, decreased lymphocytes, decreased hemoglobin, increased fasting glucose, nausea, .
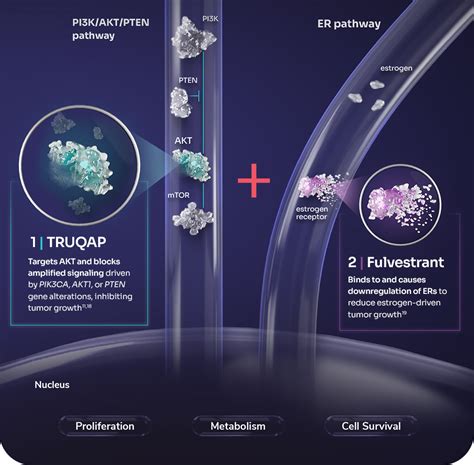
INDICATION AND USAGE. TRUQAP in combination with fulvestrant is indicated for the treatment of adult patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative locally advanced or metastatic breast cancer with one or more PIK3CA/AKT1/PTEN alteration as detected by an FDA-approved test following .Capivasertib INDICATION AND USAGE. TRUQAP in combination with fulvestrant is indicated for the treatment of adult patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative locally advanced or metastatic breast cancer with one or more PIK3CA/AKT1/PTEN alteration as detected by an FDA-approved test following .
resume TRUQAP at same dose. If recovery occurs in > 28 days, resume TRUQAP at one lower dose. FG 251-500 mg/dL: or. Withhold TRUQAP until FG decrease : ≤ 160 mg/dL (or ≤ 8.9 mmol/L). FG 14-27.8 mmol/L: If recovery occurs in ≤ 28 days, resume TRUQAP at one lower dose. If recovery occurs in > 28 days, permanently discontinue TRUQAP. FG .
Nob 17, 2023 — Truqap is the first AKT inhibitor to cross the FDA finish line. But the biomarker restriction along the PI3K-AKT pathway comes as a disappointment given that the AZ combination showed a .Nob 16, 2023 — Dosage Modifications for Strong and Moderate CYP3A Inhibitors. Avoid concomitant use with strong CYP3A inhibitors. If concomitant use with a strong CYP3A inhibitor cannot be avoided, reduce the dosage of TRUQAP to 320 mg orally twice daily for 4 days followed by 3 days off [see Drug Interactions (7.1)].. When concomitantly used .Nob 21, 2023 — Truqap is a kinase inhibitor that works by blocking pathways that help the cancer cells survive and grow, so reduces cancer growth. Truqap is from a class of medicines called an AKT inhibitor. Truqap received FDA approval on November 17, 2023, after positive results from the CAPItello-291 Phase III trial. How well does Truqap work?truqap moa Capivasertib TRUQAP (capivasertib) Jump To Highlights of Prescribing Information SPL product data elements section. 1 INDICATIONS AND USAGE. 2 DOSAGE AND ADMINISTRATION. 3 DOSAGE FORMS AND STRENGTHS. 4 CONTRAINDICATIONS. 5 WARNINGS AND PRECAUTIONS .

Truqap - Capivasertib Tablets. What is this medication? CAPIVASERTIB (kap EYE va SER tib) treats breast cancer. It works by blocking a protein that causes cancer cells to grow and multiply. This helps to slow or stop the spread of cancer cells.
/p> Description for Truqap. TRUQAP (capivasertib) is a kinase inhibitor. The molecular formula for capivasertib is C 21 H 25 ClN 6 O 2 and the molecular weight is 428.92 g/mol. The chemical name of capivasertib is 4-amino-N-[(1S)-1-(4chlorophenyl)-3-hydroxypropyl]-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-4-piperidinecarboxamide.Capivasertib is a white to off .Nob 29, 2023 — About: Capivasertib (Truqap™) Capivasertib is a kinase inhibitor. A kinase is an enzyme that promotes cell growth. There are many types of kinases, which control different phases of cell growth. Capivasertib interferes with the growth and spread of cancer cells by inhibiting PIK3CA/AKT1/PTEN. By inhibiting these proteins, this medication can .
truqap moaWILMINGTON, Del., November 17, 2023--AstraZeneca’s TRUQAP™ (capivasertib) in combination with fulvestrant has been approved in the US for the treatment of adult patients with hormone receptor .
Abr 1, 2022 — 1. Introduction. The phosphatidylinositol-3-kinase (PI3K)/Akt (PI3K/AKT) pathway is the most commonly altered signaling pathway in human cancers [].Alterations in the PI3K/AKT/mTOR pathway were identified in 38% of cancer patients including mainly phosphatase and tensin homolog (PTEN) (30%), followed by mutations in PIK3CA (13%) .
Hun 20, 2024 — Truqap Truqap is a first-in-class, potent, adenosine triphosphate (ATP)-competitive inhibitor of all three AKT isoforms (AKT1/2/3). Truqap 400mg is administered twice daily according to an intermittent dosing schedule of four days on and three days off. This was chosen in early phase trials based on tolerability and the degree of target .
Capivasertib (Truqap™) is an orally available, small-molecule pan-AKT inhibitor being developed by AstraZeneca for the treatment of various cancers, including breast and prostate cancers. Capivasertib received its first approval, in the USA, in November 2023 for use in combination with fulvestrant f .
FDA label information for this drug is available at DailyMed. Use in Cancer. Capivasertib is approved to be used with fulvestrant to treat:. Breast cancer that is hormone receptor positive and HER2 negative and has an abnormal PIK3CA, AKT1, or PTEN gene. It is used to treat adults whose breast cancer has spread and has gotten worse during or after .
On November 16, 2023, the Food and Drug Administration approved capivasertib (Truqap, AstraZeneca Pharmaceuticals) with fulvestrant for adult patients with hormone receptor (HR)-positive, human .Nob 17, 2023 — TRUQAP. TRUQAP™ (capivasertib) is a first-in-class, potent, adenosine triphosphate (ATP)-competitive inhibitor of all three AKT isoforms (AKT1/2/3). TRUQAP 400mg is administered twice daily according to an intermittent dosing schedule of four days on and three days off.
Nob 23, 2023 — Hyperglycemia: Evaluate blood glucose levels prior to starting and at regular intervals during treatment. Withhold, reduce dose, or permanently discontinue TRUQAP based on severity. (2.2, 2.4, 5.1)Diarrhea: TRUQAP caused diarrhea in most patients. Advise patients to increase oral fluids, start antidiarrheal treatment, and consult with a .
Play Free Slot-Style Social Casino Games at HollywoodCasino.com. These games are intended for use only by those 21 or older, and only for amusement purposes. No actual money or anything of value can be won playing these games. Practice or success at social casino gaming does not imply future success at real-money gambling. Gambling Problem?
truqap moa|Capivasertib